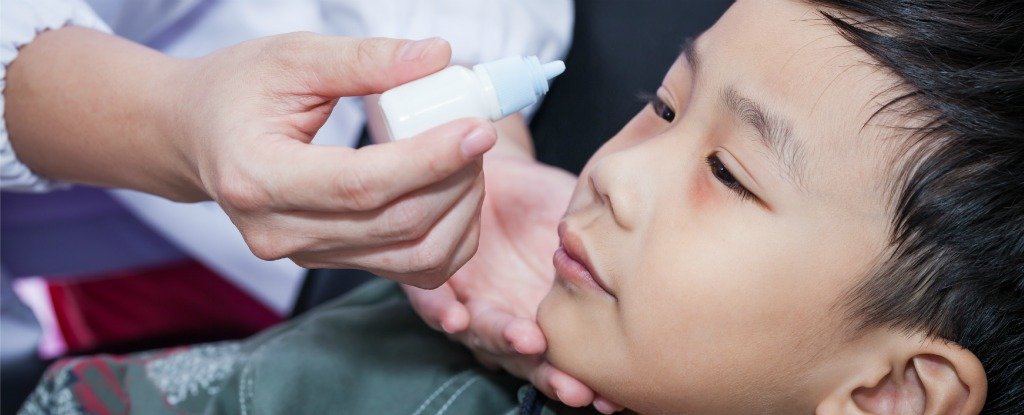
Nearsightedness Progression in Children Slowed Down by Medicated Eye Drops
Researchers present findings on five-year clinical trial of low-dose atropine for myopia at AAO 2015, the American Academy of Ophthalmology’s annual meeting
Researchers say medicated eye drops may be the key to fighting rapidly worsening eyesight in children with myopia. Results from a five-year clinical trial show that drops of low-dose atropine significantly slowed the progression of nearsightedness in children with fewer side effects than higher dosages. The research is being presented today at AAO 2015, the 119th annual meeting of the American Academy of Ophthalmology. The findings suggest that this medication could potentially be an effective treatment in the fight against the global surge in nearsightedness.
Nearsightedness, or myopia, has increased dramatically worldwide over the last few decades and remains a leading cause of visual impairment globally. In the United States, an estimated 42 percent of the population is myopic, up from 25 percent in the 1970s.1 Developed Asian countries report myopia rates of 80 to 90 percent among young adults. 2 While vision can be corrected by glasses or contacts, severe nearsightedness has ramifications that include greater risk of retinal detachment, macular degeneration, premature cataracts and glaucoma.
To help combat this public health issue, investigators in Singapore turned to atropine, a treatment commonly used to treat lazy eye. In this study, which began in 2006, 400 children age 6 to 12 were randomly assigned a daily dose of atropine. Three different groups took drops nightly at concentrations of 0.5, 0.1, or .01 percent for two years. Doctors then stopped the medication for 12 months. For children whose eyes became more myopic during that year off (-0.5 D or more), researchers started another round at 0.01 percent for another two years. The researchers discovered the following key findings:
- After five years of usage, children using the low-dose 0.01 percent atropine drops were the least myopic when compared to patients treated with higher doses.
- Atropine eye drops at 0.01 percent slowed myopia progression by an estimated 50 percent compared to children not treated with the medication in an earlier study.
- Atropine at .01 percent appears to be safe enough to use in children age 6 to 12 for up to five years, though more study is needed. The lower dose caused minimal pupil dilation (less than 1 mm), which minimized light sensitivity experienced at higher concentrations. Patients also experienced minimal near-vision loss with the low-dose drops.
Atropine inhibits axial growth of the eye associated with nearsightedness. But, the way the medication works remains largely unknown. In addition, the medication has several side effects when given at higher concentrations. For instance, at the concentration used for lazy eye, atropine dilates the pupils. This results in light sensitivity and blurry vision when looking at objects up close. Children taking higher concentrations often need to wear bifocals and sunglasses. In addition, higher concentrations have also caused allergic conjunctivitis and dermatitis.3 These drawbacks explain why atropine use for myopia to date remains fairly uncommon in the United States.
This trend could change now that much lower doses of atropine appear to offer the similar benefit in reducing nearsightedness progression, without the side effects. The researchers say this latest five-year follow-up study shows the long-term benefits outweigh the risks. However, they emphasize more information will be needed to establish which children are good candidates, as about 9 percent of children in the low-dose group did not respond to the drops in the first two years. More research is also needed to determine when treatment can safely begin and how long the drops should be used. Additional studies on atropine for myopia progression to be conducted in Europe and Japan may help find those answers.
“For a long time we’ve known that atropine drops can help keep myopia from getting worse to some degree,” said Dr. Donald T. Tan, FRCS, FRCOphth, lead investigator and professor of ophthalmology at the Singapore Eye Research Institute and the National Singapore Eye Centre. “We now have data showing that it is not only effective, but also safe. Combined with other interventions, this treatment could become a great ally in preventing myopia from causing serious visual impairment in children worldwide.”
Members of the media who would like a copy of the paper or wish to speak to an expert about the findings should contact the American Academy of Ophthalmology Public Relations Department at media@aao.org. The final results will be published in print February 2015 in Ophthalmology, the official journal of the Academy.
Five-Year Clinical Trial on Atropine for the Treatment of Myopia 2: Myopia Control with Atropine 0.01% Eyedrops will be presented at AAO 2015, the 119th annual meeting of the American Academy of Ophthalmology. Known as the place "Where all of Ophthalmology Meets,"™ the Academy’s annual meeting runs from Nov.13-17 at the Sands Expo/Venetian in Las Vegas. It is the largest ophthalmology conference in the world. For more information, see the AAO 2015 highlights.